Abstract
Litter decomposition in terrestrial ecosystems has a major role in the biogeochemical cycling of elements in the environment. Climatic features, like temperature, rainfall, humidity, and seasonal variations affect the rate of litter decomposition. This review attempts to understand the litter decomposition process in tropical forest ecosystems. It also reviews the influence of various factors on litter degradation and techniques used for assessing leaf litter decomposition. It is observed that very few studies were conducted on litter decomposition in forest ecosystems, such as tropical and temperate forests. Hence, comprehensive studies on litter degradation have to be undertaken in order to understand the turnover rate of nutrients and other elements in these sensitive ecosystems.
Similar content being viewed by others
1 Introduction
Litter fall in terrestrial ecosystems signifies a crucial pathway for nutrient return to the soil. Leaf tissue can account for more than 70% of above ground litter fall in forests, and the rest is composed of stems, small twigs and propagative structures (Robertson and Paul 1999). “Litter mass loss” or “decay” is the sum of carbon dioxide (CO2) release and discharge of compounds, which contains both carbon compounds and nutrients (Brady and Weil 2010). Litter decomposition proceeds through numerous mechanisms, especially heterotrophic consumption of organic composites in litter (Bezkorovainaya 2005). Rainwater leaching and the activities of small insects do not lead straight to CO2 release to the atmosphere, even though they support litter decomposition. The CO2 released through microbial decomposition can add more than 20% to soil surface CO2 efflux, which is known as soil respiration. In advance, nitrogen (N), phosphorus (P) and calcium (Ca) released from plant litter through decomposition are accessible for plants and microbial uptake. This review summarises the role of microbes and plants in the litter degradation process and also the importance of nutrient cycling and the mineralisation process (Ball 1997).
2 Litter
Ecologically the term litter has two meanings: the layer of dead plant material present on the soil surface or dead plant material that is detached from a living plant. The litter strata can be different from the mineral layer but this is not true for the layer comprising of identifiable plant materials and the layer encompassing merely amorphous organic material (Anderson and Ingram 1983). There is no benchmark for the beginning of decomposition of litter that is detached from the living plant. A dead branch in the crown of a tree may have decomposed to its partial live weight before it drops to the ground, and the heartwood of a tree may die and decompose wholly before the tree falls (Bremer et al. 1991). The presence of a large amount of litter on the forest floor has a significant influence on forest ecosystem dynamics (Olsen 1963).
3 Litter decomposition
Litter decomposition plays a vital part in the nutrient budget of a forest ecosystem, where flora is influenced most significantly by nutrient recycling from plant litter (Vesterdal 1999; Wedderburn and Carter 1999). Litter decomposition encompasses ample breakdown of organic matter into CO2 and nutrients via physical, biological and chemical pathways (Aerts 1997). It returns carbon, as CO2, into the atmosphere through the heterotrophic respiration of soil microorganisms and animals (Chandrasekhara 1997; Schimel 1995; Wachendorf et al. 1997).
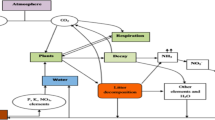
Slow decomposition rates result in the building up of organic matter and nutrient stocks in soil; however, fast decomposition rates help to meet plant intake requirements (Isaac and Nair 2005). Climatic features, such as temperature, rainfall and seasonal variations, may influence the existence of microbes and other soil fauna that significantly affect the rate of decomposition. The litter diversity also influences the activity of soil communities and processes during decomposition (Chapman and Koch 2007). The ecosystem significance of a variety of soil organisms is poorly understood, except for earthworms, termites and ants (Jones et al. 1994; Anderson 1995). A schematic representation of litter degradation is shown in Fig. 1.
4 Factors affecting litter decomposition
Litter decomposition consists of two simultaneous processes: (a) the associated mineralisation and humification of lignin, cellulose and other compounds through a series of actions by microorganisms and (b) the leaching of soluble compounds into the soil whose carbon and nitrogen are gradually mineralised (Anderson 1988). These methods depend on abiotic factors like temperature, humidity and biotic features, such as chemical composition of litter and soil organisms (Aber and Melillo 1982). Hence, the physico-chemical environment, litter quality and the composition of the decomposer community are the three leading features regulating litter decomposition (Berg et al. 1993; Couteaux et al. 1995; Cadish and Giller 1997; Bohlen et al. 1997; Dechaine et al. 2005).
Temperature can be considered as a prime factor in determining the rates of litter decomposition (Meentemeyer 1978; Hobbie 1996), and decomposition is more sensitive to temperature than the primary production (Lloyd and Taylor 1994; Kirschbaum 2000). Soil microbial activity rises exponentially with soil temperature (Kirschbaum 1995). A few studies have indicated the role of the chemical nature of the litter in decomposition along with climate (Swift et al. 1979; Berg et al. 2000).
Fresh leaf litter is a readily available substrate for soil macro- and microfauna. The litter quality also affects the degradation process, as it generally reduces throughout the decomposition due to the loss of readily accessible carbon and the accumulation of recalcitrant compounds (Dilly and Munch 2001; Rosenbrock et al. 1995). Liu et al. (2010) revealed the influence of the type of leaf litter on the decomposition process and soil microbes (Coleman and Crossley 1996). The major factors that influence litter degradation are diagrammatically represented in Fig. 1.
5 Role of soil properties
Soil physical and chemical characteristics have a significant role in litter decomposition. Among them, texture is the most significant as it stimulates nutrient and water dynamics, porosity, permeability and surface area. The major chemical properties include pH, cation exchange capacity, organic matter content and nutrients (Coleman et al. 1999). The organic matter, which influences the different physico-chemical factors like bulk density, pH, is the major soil property affecting litter decomposition (Cuevas and Medina 1986). The organic matter can also increase the population density of soil macroorganisms, which plays a significant role in litter mixing and decomposition (Akpor et al. 2006). Among the mineral nutrients, soil nitrogen status is deliberated as being the primary regulating factor and has received utmost attention, while phosphorous is usually considered as a limiting nutrient because of the low quantity in circulation in major forests. Calcium, nitrogen and phosphorus are rapidly mineralised in litter (takes several weeks/months), but organic complexes in the soil organic matter pools have much slower turnover times, taking several years or decades (Devi and Yadava 2007). However, while considering an entire decay process, the effects of added nitrogen on the rate of decomposition seem to be irrelevant and can even turn out to be contrary (Fog 1988).
Potassium and magnesium are essential nutrients for higher plants but hardly limit the microbial actions and are easily removed from decomposing litter (Anderson and Ingram 1983). Nutrient cycles in rain forests differ with soil type, climate and topographic locations; hence, the moisture content and temperature are also unavoidable factors in the litter degradation process (Esperschutz et al. 2011).
6 Role of trees and litter quality
The major component of organic material in forest soil results from the vegetation that is deposited on the soil surface as an organic layer (litter) and is partially dispersed into the soil (Klein and Dutrow 2000; Santa Regina and Tarazona 2001).
Plant litter contains various classes of organic compounds. There are four major assemblies of soluble organic material in litter: sugars, phenolics, hydrocarbons and glycerides. The soluble sugars, primarily mono and oligosaccharides are difficult to metabolise. The relative proportions of these compounds differ with the plant part (leaves, stems, roots, bark) and plant species. The plant litter quality is measured by means of chemical composition of nitrogen, phosphorus, potassium and chief cell wall components, such as lignin, cellulose and hemicelluloses that influence the litter decomposition and nutrient release (Swift et al. 1979).
Lignin accounts for about 15–40% of the total litter quantity. In certain extreme cases, litter can have lignin contents as low as 4% or as high as 50%. Lignin, in contrast to cellulose, is an extremely flexible molecule. The structure of lignin differs with the plant species. For example, deciduous species are comprised of fluctuating proportions of syringyl and guaiacyl forms of lignin, while conifers have generally guaiacyl lignin (Esperschutz et al. 2013).
In addition to lignin, the carbohydrates, such as cellulose and hemicelluloses, are the common constituents in plant litter in terms of quantity. Of these, cellulose (10–50% of the litter quantity) is made up of glucose elements linked with β-1-4 bonds that create long chains of molecules organised into fibres. Hemicelluloses are polymers of sugars like glucose, and the amounts of these may differ among litter species (Akpor et al. 2005). The ratios of hemicelluloses to cellulose range from 0.7 to 1.2; upper ratios are frequently perceived in deciduous litter (e.g., beech) and the lower ratios in coniferous litter (e.g., spruce) (Fengel and Wegener 1983).
Litter decomposition rates vary widely among species that decompose in identical ecological situations (Cornelissen 1996; Wardle et al. 1997). These alterations in decomposition are mainly due to differences in litter traits, such as leaf toughness, nitrogen, lignin, polyphenol concentrations, the C/N ratio and lignin/nitrogen ratio (Berg et al. 1993; Cadish and Giller 1997; Perez-Harguindeguy et al. 2000). Among the various traits, nitrogen and lignin content of plant material are the most significant in regulating the rates of decomposition (Millar et al. 1936; Minderman 1968; Fogel and Cromack 1977; Gartner and Cardon 2004; Meentemeyer 1978). On the basis of the close association between litter quality and decomposition, litter traits can be used as forecasters for decay rates between species (Aber et al. 1990) and also serve as important variables in biogeochemical models (Nicolardot et al. 2001).
Litter quality typically reduces throughout decomposition due to the loss of easily attainable carbon and the accumulation of recalcitrant compounds (Gaudinski et al. 2000). The leaves of coniferous trees decay more slowly than those of deciduous trees, as broad-leaved litter covers more potassium and phosphorus, less lignin and nearly always less ether-soluble sections (Daubemire and Prusso 1963; Gosz et al. 1973; Mikola 1960; Ovington 1954). Alterations among hardwood species remain substantial (Edwards and Heath 1963). The decomposition of teak litter was faster than that of Acacia arabica litter; moreover, leaf litter vanishes much sooner than twigs and branches (Rochow 1974) and litter under forest canopy is softer and disappears more quickly than leaves exposed to sunlight (Giller and Gadisch 1997; Willams and Gray 1974). Deviations in the rate of leaf litter decomposition of the same plants during different seasons at different locations are also observed (Kumar et al. 2012). Studies show that climatic variations could be a major reason for this, as this is known to be the leading factor influencing litter decomposition on a large geographic scale (Meentemeyer 1978; Dyer et al. 1990; Austin and Vitousek 2000).
The rate of decomposition is high in species with extreme ash and nitrogen contents and the lowest C/N ratios and lignin contents. Species showing average ash, nitrogen and lignin contents and a normal C/N ratio appears to decay at a transitional rate. Kucera (1959) reported a progressive correlation between both the rate of decay and ash content of hot-water-soluble materials (Gonzalez and Seastedt 2001).
The concentrations of nutrients vary with the litter species. For instance, leaf litter of the nitrogen fixative genus alder (Alnus) has great actual concentrations of N (often above 3%); in contrast, pine needle litter is nitrogen poor (frequently under 0.4%). Plant species is therefore a prevailing feature in defining the litter value (Gustafson 1943; Berg and McClaugherty 2003).
7 Role of soil fauna and microbes
The abundance and arrangement of soil fauna and microbial populations are known to affect the rate of litter breakdown at various stages of decomposition (Schaefer and Schauermann 1990; Dilly et al. 2004). Microbial decomposition of organic material on the forest soil has a significant effect on soil carbon and energy flow in the ecosystem. The variety of such soil microbes is supposed to be extremely high; however, they are mainly anonymous (Prosser 2002). Species variety of soil fungi is slightly lower than that of bacteria (Bridge and Spooner 2001; Hawksworth 2001), due to their high productivity and fast growth (Hanson et al. 2005). The count of bacterial species is in the order of hundreds to thousands in 1 g of soil, whereas entire species number is more than 2–3 million (Torsvik et al. 1994; Dejonghe et al. 2001; Prescott et al. 2000).
Among the soil microfauna, fungi are the leading decomposer and have more than 75% greater potential to reduce organic matter than other microorganisms (Kjoller and Struwe 1992). Furthermore, their activity will vary seasonally. Besides fungus, litter bacteria are a significant part of the process of organic matter mineralisation and accounts for 25–30% of the total soil microbial biomass (Dilly and Munch 2001; Kurihara and Kikkawa 1986; Persson 1980).
Leaf decomposition by fungi and bacteria tends to be rapid at nutrient-enriched conditions. The involvement of fungi and bacteria in leaf decomposition could react inversely to stress situations (Pascoal and Cassio 2004). Microbes can also be limited by soil moisture. As the temperature rises, soil moisture has a progressively more significant role in retaining high rates of microbial activity (Peterjohn et al. 1994). As a result, the rate of fresh litter decomposition rises with both increasing temperature and precipitation (Meentemeyer 1978).
The growth of microbes, especially fungi, on the litter may initiate decomposition prior to litter fall; however, the growth of decomposers only takes place when the litter reaches the floor. The arrangement of the microbial community that occupies the litter depends on the properties of the litter, soil features and variations of these properties over time (Harmon et al. 1999).
The role of numerous classes of bacteria and fungi in litter degradation was recognised in earlier studies (Table 1) and showed that, under laboratory conditions, forest soil and related microbial communities act as vital variables in litter decomposition process (Frankland 1992; Rosenbrock et al. 1995; Cox et al. 1997, 2001; Prescott 1996; Chadwick et al. 1998). Litter decomposition is also influenced by the quantity and quality of litter input, which is dependent on plant species (Chadwick et al. 1998; Hattenschwiler et al. 2005).
Besides fungi and bacteria, soil biota comprises of both micro- and macroinvertebrates (Heath 1966). Microarthropods, which survive in the litter strata and on the upper layer of the soil, are an essential part of the ecosystems due to their significant role in organic matter decomposition and mineralisation processes, nutrient cycling (Irmler 1982) and pedogenesis. Soil faunal activities mainly help to acclimatise the litter and motivate microbial activity. The labile compounds (e.g., sugars, amino acids) in litter may be absorbed by soil microbes and therefore prone to rapid decay (Hobbie 1996). The labile structural compounds, such as cellulose, are quickly cleaved by exo-enzymes into sugar sub-units, which again are readily absorbed by microbes. In contrast, refractory structural compounds, such as lignin and chitin, are too large to pass through cell membranes and remain unchanged to extracellular enzymes due to their uneven chemical structure and complex bonding (Horner et al. 1988).
Studies suggested that the presence of fauna on the leaf discs and the leaf tissue consumption was less during winter (Crossley and Hoglund 1962; Madge 1965). Edwards and Heath (1963) noticed that earthworms were able to decompose litter three times faster than minor invertebrates, such as springtails, enchytraeids and larvae (Jenkinson et al. 1994).
8 Degradation patterns of major polymers in litter
The degradation patterns of major polymers in litter are given in Table 2.
8.1 Cellulose
Cellulose in the plant fibre is organised in a crystal-like form that makes it hard to attack. Cellulose is decomposed using extracellular enzymes by both bacteria and fungi. It is first degraded to monomers, or oligomers of a rare glucose unit, such as cellobiose, which can be engaged into the microbial cell and metabolised (Johansson 1994a, b). Various organisms are capable of degrading the more amorphous kind of cellulose (Eriksson et al. 1990). The wood-decay fungus, white-rot basidiomycete (Phanerochaete chrysosporium), has been used for deterioration of lignocellulosic constituents (Tien and Kirk 1984; Higuchi 1993).
Three major hydrolytic enzymes carry out cellulose degradation: endo-1, 4-glucanase shelters the cellulose chain and ruptures the glucosidic relations via a random method. Exo-1, 4-glucanase ruptures either cellobiose or glucose from the non-reducing end of the cellulose chain. Finally 1, 4-glucosidase hydrolyses cellobiose and further water-soluble oligosaccharides, such as triose and tetrose, to glucose. These enzymes are dissimilar in nature and have different specificities (Johnson and Catley 2002). The endo and exoglucanases have a synergistic action that allows them to decompose crystalline and amorphous cellulose. In addition to hydrolytic enzymes, certain cellulolytic entities yield cellobiose dehydrogenase, which is found in a variety of fungi and seems to have a role in lignin and cellulose degradation (Kelly and Beauchamp 1987).
The soft-rot fungus seems to have a cellulose-degrading scheme like that of the white rots. Brown rots have not yet been observed to require the synergistic enzymes that are found in white rots and they do not have the exoglucanase. Highley (1988) found numerous species of brown rots that were able to solubilise microcrystalline cellulose. These fungi simply depolymerise cellulose, without producing soluble monomers or dimers. Still, no additional enzyme has been found to account for the lost exoglucanase that splits off from the soluble components. Hence, Eriksson et al. (1990) suggested a non-enzymatic mechanism.
Comprehensive studies on Clostridium cellulolyticum illustrate that the organism yields at least six dissimilar cellulases, each one with diverse structural and catalytic properties (Klein and Dutrow 2000). Both cellulases and xylanases are held together in a huge arrangement, known as the cellulosome, by a platform protein, as proposed by Eriksson et al. (1990). Earlier, the formation of the cellulosome itself was observed in an anaerobic bacterium Clostridium thermocellum (Viljoen et al. 1926).
The degradation of cellulose by bacteria is suggested to be hydrolytic, while the mechanisms seem to be different from those found in fungi. For bacteria, the cellulolytic enzymes are organised in groups and perform via a collective method (Knapp et al. 1983). There are small additional groups of cellulolytic bacteria, comprising Cytophaga, Cellulomonas, Pseudomonas and Cellvibrio. It seems that these bacteria have their cellulolytic enzymes bound to the cell wall and, consequently, an adjacent connection is necessary between the cell and the substrate (Berg et al. 1972; Eriksson et al. 1990; Wiegel and Dykstra 1984).The major bacteria that are capable of utilising cellulose are Achromobacter, Angiococcus, Bacillus, Cellfalcicula, Cellulomonas, cellvibrio, Clostridium, Cytophaga, Polyangium, Pseudomonas, Sorangium, Sporocytophaga, Micromonospora, Nocardia and Vibrio (Krivtsov et al. 2005).
Actinomycetes degrade the cellulose in a manner similar to that of fungi and can also degrade the crystalline form. Several strains have the ability to degrade the lignocellulose complex (Wang et al. 1999). Actinomycetes, like Actinokineospora, Streptomyces, Nocardiodes, Pseudonocardia, Nocardia and Micromonospora, are capable of decomposing plant litter (Das and Battles 2007). The mode of enzymatic degradation of cellulose and also the lignocellulose complex of actinomycetes is similar to that of fungi (Finlay et al. 2000). The production of cellulases is influenced by cellulose, cellobiose, sophorose and lactose (Lueken et al. 1962). The existence of cellulose seems to be the best stimulation agent, whereas glucose suppresses the production of the cellulase system (Wood 1995). As cellulose is a large and non-soluble molecule, it cannot be absorbed into the microbial cells for a persuading effect to be applied. Currently, the conventional theory is that the entities have a constant, rudimentary level of cellulase on their surface (Mahasneh 2001). Upon connection with cellulose, small quantities of persuading materials are released from the cellulose; these enter into the microbial cell and influence cellulose creation. It is expected a little intracellular absorption of a type of compound resembling cellobiose or cellotriose can stimulate the production of cellulose (Martin and Marinissen 1993).
8.2 Hemicelluloses
In wood, the entire absorption of hemicelluloses typically ranges from 20 to 30%. There is variance in the structure and arrangement of hemicelluloses in litters of softwood as compared to hardwood (Wolter et al. 1980). The hemicelluloses are composed of both linear and branched heteropolymers of d-xylose, l-arabinose, d-mannose, d-glucose, d-galactose and d-glucuronic acid and are individually methylated or acetylated (McTiernan et al. 1997).
Degradation of hemicelluloses requires additional composite enzyme systems for the hydrolysis of cellulose. The degradation of such a molecule requires the concentrated action of several hydrolytic enzymes (Eriksson et al. 1990). The major bacterial species involved in the utilisation of hemicellulose are Bacillus, Achromobacter, Pseudomonas, Cytophaga, Sporocytophaga, Lactobacillus, Vibrio and Streptomyces (Mikola 1973).
Major starch-utilising bacteria are Achromobacter, Bacillus, Chromobacterium, Clostridium, Cytophaga, Micromonospora and Nocardia, whereas protein-using bacteria include Bacillus, Pseudomonas, Clostridium, Serratia and Micromonospora.
8.3 Pectin
Pectin is a highly methylated form of poly-1,4-D galacturonic acid. E. chrysanthemi and E.cartovora are induced to form a complex of enzymes that constitute the degradation of pectin. Initially pectin is demethylated to pectic acid (polygalacturonate) by pectin methyl esterase (Nye 1961). Enzymes, such as pectatelyase (an endo pectatelyase), exo polygalacturonase and oligo uronidelyase, are involved in the degradation of pectin. Enzymes that degrade pectin or poly galacturonic acid are found in Pseudomonas, Bacillus, Clostridium, Lachospina, Butyri vibrio and Bacteroides (Ovington and Madgwick 1957).
8.4 Lignin
Lignin degradation is considered to vary between the three common sets of decomposers: white-rot, soft-rot and brown-rot fungi. The diverse enzymatic mechanisms of lignin degradation are merely defined, except for Phanerochaete chrysosporium, which is a white-rot fungus (Rigobelo and Nahas 2004).
9 Lignin degradation by white rot fungi
White-rot fungi have the capability to completely mineralise lignin to CO2 and water. The outcome, for wood, is that the whole lignocellulosic complex is decayed more or less instantaneously. A bulky cluster of the white rots might even decompose lignin differently to cellulose (Hatakka 2001). The attack of lignin arrangement has long been supposed to start through the elimination of the methoxyl group. An earlier investigation revealed that a mixture of hydroxylation and demethylation is shadowed by an oxidative attack on the aromatic ring (Eriksson et al. 1990).
Lignolytic schemes are species specific and depend on the ecological niche of the fungus (Hatakka 2001). For instance, the white-rot Ganoderma lucidum creates Mn-peroxidase in a medium with popular wood; however, it does not in pine (D’Souza et al. 1999). Such interpretations might support the outcome that white-rot fungi are usually found on angiosperm than on gymnosperm woods (Gilbertson 1980).
10 Lignin degradation by brown-rot fungi
Brown-rot fungi mostly decay the cellulose and hemicellulose constituents in wood and have the capability to adapt the lignin molecule (Eriksson et al. 1990). Brown-rot and white-rot fungi have similar decomposition mechanisms whereby hydroxyl radicals are created that attack the wood constituents (Hatakka 2001). It is expected that all brown-rot fungi use a similar mechanism for wood decay. The initiation of the decomposition of lignin and cellulose together seems to be through diffusible minor molecules that can pierce the cell wall. In contrast to white rots, only brown rot is set up to create Mn-peroxidase (Sarah 1996). The radicals made by brown-rot fungi can eradicate methoxyl groups from lignin and yield methanol, leaving the remains of mostly altered lignin (Eriksson et al. 1990) where the presence of phenolic hydroxyl groups is high (Crawford 1981). Carbonyl and carboxyl groups are also produced (Jin et al. 1990). Hence brown-rotted lignin remains more responsive than natural lignin.
11 Lignin degradation by soft-rot fungi
The literature suggests that soft-rot fungi do not decompose lignin; however, it does soften wood by breaking down the middle lamella of the cell wall. Most soft-rot fungi are ascomycetes and deuteromycetes and are most lively in moist wood (Scholle et al. 1992). Crawford (1981) observed that soft-rot fungi remained capable of reducing the lignin content in decomposing wood. Another study showed that soft-rot fungi decompose lignin up to 44% under laboratory conditions (Nilsson et al. 1989). The lignolytic peroxidases of soft-rot fungi do not have the potential to oxidise the softwood lignin, which has a high level of guaiacyl components.
12 Microbial litter decomposition and biogeochemical cycling
The deposition of carbon into the soil is a significant part of carbon cycling in terrestrial ecosystems. The chemical components of the litter are organised and reabsorbed by plant roots, resuming a novel plant nutrient cycling and assuring recurrent situations to the system (Guo and Sims 1999). The major aspects that govern the organic matter conversion are the quantity and quality of litter material constituents, the physical and chemical environment and the decay entities (Swift et al. 1979). The rate of decomposition and nutrient dynamics of leaf litter are influenced by the arrangement of decomposers in the soil (Swift et al. 1979). The bacterial community, their respiratory action and particular soil chemical complexes designate the transformation development that occurs in soils under exact forest litters (Luizao et al. 1992). Furthermore, edaphic and climatic features affect the action of soil microbial enzymes (Jha et al. 1992). It is expected that the nutrients released during litter decay can account for 67–87% of the annual demand for forest plants (Waring and Schleslnger 1985). The litter decomposition is closely related to microbial activities that modify the litter chemical composition and regulate carbon and nitrogen dynamics in soil. The development of microbes, as well as the subsequent biomass and necromass, considerably changes the chemical features of soil organic matter, as detected in nutrient immobilisation (Simpson et al. 2007). The role of litter decomposition in the biogeochemical cycle is illustrated in Fig. 2.
Role of litter degradation in biogeochemical cycles (the various elements and compounds accumulating in plants return into the environment through litter degradation where these substances leached into the soil and diffused into the atmosphere. Hence, these substances are again getting into the biogeochemical cycle)
12.1 Carbon cycle
Microbial biomass consists of less than 35% of the total organic carbon in soils (Schlesinger 1997). The decay rate of humus in a natural forest environment is lower than that of an agricultural field. Depending on the substrate value, carbon complexes can be broken down by the enzymatic action of microbes. In forest soils, the decay of leaf litter yields high amounts of dissolved organic carbon compounds (Singh and Gupta 1977). About 5–40% of the whole carbon losses may be due to leaching. On the other hand, a lesser amount of carbon is removed by soil erosion under forest cover. This acts to reduce the decay ratio and total stored carbon in the soil. Johnson and Curtis (2001) revealed that the elimination of saw-log forest tended to increase the quantity of carbon and nitrogen in the soil for a small duration. This is due to the fast assimilation of minor size carbon material into the soil, which facilities microbial decay of the carbon molecules and discharge of the excess nutrients towards the soil (Swarnalatha and Reddy 2011).
12.2 Nitrogen cycle
The adsorbed and complexed nitrogen will be remobilised from the sources by microbes. The mineralised nitrogen is recovered and consumed by plant roots or recycled by the micro-flora when microbes die. Merely 1–3% of the organic nitrogen in soil is mineralised during its development (Bartholomew and Kirkham 1960).
In soils, nitrogen is associated with the soil organic material, which contains about 5% of the total nitrogen (Brady and Weil 2010). This organic nitrogen is not available for plants, so the microorganisms decay the organic matter into smaller particles through the discharge of ammonium. The mineralisation of organic nitrogen compounds in natural forest soils is a gradual process and is commonly facilitated by some degree of microbial activity because of the lower availability of organic nitrogen. As such, soil nitrogen cannot be considered as a chief nitrogen pool (Teuben 1991).
The limitations in the degradation of nitrogenous compounds are indicated in previous studies and are: (a) polyphenols, amino acids and additional nitrogenous materials are reduced into small molecules with a comparatively smaller surface available for enzyme action, (b) the physical sorption of humus by clay reduces the dynamic groups of the humus protein that is unreachable to microbial proteases, (c) much of the soil organic material that is placed inside the soil pore spaces is too small to be available for microbes, and (d) in the deadly phases of decomposition, the arrangement of humic molecules is so uneven that there is lower possibility of specific enzymes meeting the particular bonds (Black 1968; Tripathi et al. 2010).
12.2.1 Phosphorous
Phosphorus is next to nitrogen as a regulating nutrient. Like nitrogen, the phosphorous concentration in litter increases during decomposition. The initial concentration is decreased due to leaching. Litter decomposition provides a very small concentration of orthophosphate to plants (Verhoef and Brussaard 1990). Organic acids formed by microbial decay of plant remains might collect locally to reach concentrations that can increase the accessibility of phosphates to plants. Organic phosphorus generally mineralises gradually, as specified by Mattingly and Williams (1962).
12.2.2 Potassium
The potassium from plant litter does not gather in surface horizons. However, the arrangement and amount of litter decay might affect its reachability to plants more than the influence of the remaining organic matter on the cation exchange capability of the soil. Potassium and magnesium are essential nutrients for higher plants; however, they rarely limit microbial actions and are quickly removed from decaying litter (Anderson and Ingram 1983).
The presence of tree roots running through litter on the forest floor might diminish leaching losses of nutrients via the direct uptake of K, Mg and Ca (Cuevas and Medina 1988; Medina and Cuevas 1989). There is evidence of direct acceptance of potassium from litter through roots (Herrera et al. 1978).
Loreau (2001) suggested that microbial diversity has a positive influence on nutrient cycling proficiency and ecosystem processes. Among the soil organisms, bacteria and fungi have excellent characteristics of biomass and respiratory metabolic rate and have more involvement in the organic matter decay procedure (Persson 1980). The bacterial community, their respiratory activity and exact soil chemical composites specify the transformation development occurring in soils under specific forest litters (Luizao et al. 1992).
13 C/N ratio of the plant litter and its decomposition
Reports show that leaf litter decomposition can be calculated from the C/N ratio (Melillo et al. 1982). High-quality leaves (nutrient-enriched leaves) will generally decompose more rapidly than low-quality leaves (nutrient-deficient leaves). In general, the decomposition rate is high in species with extreme ash and nitrogen contents and minimum C/N ratios and lignin contents (Singh 1969). Several works showed that the nitrogen concentration of the litter and the C/N ratio is strongly associated with litter decay rates (Berg and Staaf 1981). The concentration of phosphorous and C/P ratios appeared to be good predictors of decay rates (Vitousek et al. 1994). Concentrations of lignin and the lignin/N ratios in plant litter are also good predictors of litter decomposition (Meentemeyer 1978; Melillo et al. 1982). These factors and their effects on litter decomposition depend on soil characteristics and plant species.
14 Various techniques for assessing litter decomposition
14.1 Mass balance technique
Mass balance methods are used to evaluate litter decay in different ecosystems (Olsen 1963; Schlesinger 1997). This method assumes that a constant fraction, k, of the detrital litter quantity decays:
In forest, values for k are larger than 1.0. Environments with slow decomposition rates and low surface litter deposition have k values less than 1. Litter fall is measured by means of litterbags that are unsystematically set apart in the study location (Bubb et al. 1998; Xu and Hirata 2002).
The mass balance method can be used to evaluate litter decay, or to validate model forecasts (Hedin 2000). On the occasion that the forest floor is rapidly aggrading, the technique would over-estimate decay rates. As this technique depends on natural litter fall, this method cannot be used to efficiently explain the role of factors like temperature and moisture, as is possible with litterbag experiments.
14.2 Litterbag technique
The litterbag method is extensively used to study decay at the soil surface. Fresh leaf litter is placed in litterbags, which are then inserted into the litter layer of the soil and gathered at periodic intermissions so that the remaining quantity can be measured. Mesh size is usually selected as to increase the entry of organisms to the litter, while reducing too much particle loss. Litterbags with different mesh sizes have been used to influence the microbial composition (Crossley and Hoglund 1962). Very small mesh size will not remove certain organisms but will prevent particle loss to mineral soil as well. Fibreglass mesh has been suggested for light concentrated places, as UV light will degrade nylon and other materials (Harmon and Lajtha 1999). Yet, 1–2 mm mesh is most suitable in litterbag studies (Robertson and Paul 1999); mesh size must be more than 2 mm to permit the entry of macrofauna.
The size and content of the litterbags remains a significant constituent of litterbag studies. Litterbags of 20 × 20 cm are common (Robertson and Paul 1999) in different plant populations or where leaves are large and a larger litterbag is suitable.
14.3 Tethered leaves technique
The tethered leaf method is similar to the litterbag method. The specific leaves are tightly packed in packages somewhat located in litterbags. Either a particular leaf or a group of leaves are tied together by means of nylon thread or monofilament fishing line. The line is tied to the leaf petiole for stability; the line is typically attached to a coordination point to facilitate gathering and a tag for recognition.
A “wheel spoke” method, after Vitousek et al. (1994), is frequently used in terrestrial studies, where a group of specific senescent leaves are air-dried, again with their petioles tied to a solitary line. One end remains tied to a recognising tag and the other end to a labelled washer. Numerous sets of threads are tied to every washer in this manner.
Tethered leaf studies remain very relevant in learning the initial phases of decay; therefore, length of study is not equal as that of litterbag methods. As the leaves begin to fragment, this method will over-estimate decay rates as compared to the litterbag method. Studies revealed that litter-feeding invertebrates could attain ready contact with litter in litterbags with mesh sizes as small as 1.5 mm (Scowcroft et al. 2000). Yet, the tethered leaf method permits the leaf intake by macroinvertebrates like crabs and snails, whose contact would otherwise be restricted mesh bags (McKee and Faulkner 2000).
14.4 Cohort layered screen technique
A fourth approach to assess high leaf litter decay is the cohort layered window screen method, or litter sandwich method. By this technique, layers of mesh screen are used to separate consecutive sheets of litter on the forest surface, where the leaf litter decays in situ on the previous litter layer.
The cohort layered screen process is applied to long-term decay studies, normally of three or more years (Binkley 2002). Upon annual litter fall, a new screen window is located above the forest floor. Usually a 1 × 1 m fibreglass or aluminium window screen with a mesh size of 2–3 mm is used. The screen dimension will be determined by the size of the stand tested, and mesh size will differ depending on the exact ecology under study. A fibreglass screen is recommended over aluminium if any chemical or essential properties will be evaluated as well. During the period of study, for every consequent annual litter fall, an additional screen is positioned straight above the previous screen. Subsamples are gathered, weighed and oven dried. The comparison of different techniques used for litter degradation study shown that the litterbag technique is more appropriate method (Table 3).
15 Conclusion
Litter decomposition is highly significant in the functioning of ecosystems, as it is a major way of recycling of nutrients, especially carbon and nitrogen and other elements in the ecosystem. The degradation rate of plant material and uptake of minerals are in equilibrium in an ecosystem and vary depending on the type of ecosystems. Litter decomposition is a highly complex process that involves a number of physical, chemical and biological factors; however, there is little information about the litter decomposition rate and the role of various factors in different ecosystems. Also it is very difficult to understand the rate of litter degradation as it is influenced by a number of entirely different factors. Researchers are yet to finalise a methodology to detect the rate of litter degradation, which can incorporate all the factors. However, it is significant to study litter degradation in the context of increasing anthropogenic impacts on biogeochemical cycles. This review focuses on various factors that affect the litter degradation and degradation patterns of the various polymers in leaf litter. It also emphasised and discussed various methods for assessing litter degradation. The review found that there are very few studies on litter degradation and element recycling in various ecosystems. Hence, future research must be centralised on the following subject areas: (a) development of a methodology for assessing the rate of litter degradation; (b) litter degradation and climate change; (c) transport pathways of elements during litter degradation, etc.
References
Aber JD, Melillo JM (1982) Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can J Bot 60:2263–2269
Aber JD, Melillo JM, McClaugherty CA (1990) Predicting long term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Aerts R (1997) Climate, leaf litter chemistry and leaf-litter decomposition in terrestrial ecosystems—a triangular relationship. Oikos 79:439–449
Akpor BO, Okoh AI, Babalola GO (2005) Culturable microbial population during decomposition of Cola nitida leaf litters in a tropical soil setting 18(3):313–319
Akpor OB, Okoh AI, Babalola GO (2006) Culturable microbial population dynamics during decomposition of Theobroma cacao leaf litters in a tropical soil setting. J Biol Sci 6(4):768–774
Anderson JM (1988) Spatio-temporal effects of invertebrates on soil processes. Biol Fert Soils 6:216–227
Anderson JM (1995) Soil organisms as engineers: microsite modulation of macroscale processes. In: Jones CG, Lawton JH (eds) Linking species to ecosystems. Chapman & Hill, New York, pp 94–106
Anderson JM, Ingram JSI (1983) Tropical soil biology and fertility: a handbook of methods. CAB International, Wallingford
Austin AT, Vitousek PM (2000) Precipitation decomposition and litter decomposability of Metrosideros polymorpha in native forests of Hawaii. J Ecol 88:129–138
Ball AS (1997) Microbial decomposition at elevated CO2 levels: effect of litter quality. Glob Change Biol 3:379–386
Bartholomew WV, Kirkham D (1960) Mathematical description and interpretation of culture induced soil nitrogen changes, vol 2. In Trans 7th Int Congr Soil Sci, pp 471–477
Berg B, McClaugherty C (2003) Plant litter decomposition humus formation. Carbon sequestration. Springer, Berlin, p 296
Berg B, Staaf H (1981) Leaching accumulation, and release of nitrogen in decomposing forest litter. In: Clark FE, Rosswall T (eds) Terrestrial nitrogen cycles. Processes, ecosystem strategies, and management impacts, Ecol Bull (Stockholm) 33. pp 163–178
Berg B, von Hofsten B, Pettersson G (1972) Electron microscopic observations on the degradation of cellulose fibres by Cellvibrio fulvus and Sporocytophaga myxococcoides. J Appl Bacteriol 35:215–219
Berg B, Berg MP, Bottner P, Box E, Breyner A (1993) Litter mass loss rates in pine forests of Europe and eastern United States: some relationship with climate and litter quality. Biogeochemistry 20:127–159
Berg B, Meentemeyer V, Johansson MB (2000) Litter decomposition in a climatic transects of Norway spruce forests—climate and lignin control of mass-loss rates. Can J For Res 30:1136–1147
Bezkorovainaya IN (2005) The formation of soil invertebrate communities in the Siberian aforestation experiment. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Dordrecht, pp 307–316
Binkley D (2002) Ten year decomposition in a loblolly pine forest. Can J For Res 32(12):2231–2235
Black CA (1968) Soil-plant relationships. John Wiley and Sons Inc, NewYork, p 792
Bohlen PJ, Parmalee RW, McCartney DA, Edwards CA (1997) Earthworm effects on carbon and nitrogen dynamics of surface litter in corn agroecosystems. Ecol Appl 7(4):1341–1349
Brady N, Weil R (2010) The nature and properties of soils. Pearson, Upper Saddle River
Bremer E, van Houtum W, van Kessel C (1991) Carbon dioxide evolution from wheat and lentil residues affected by grinding, added nitrogen, and absence of soil. Biol Fertil Soils 11:221–227
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232:147–154
Bubb KA, Xu ZH, Simpson JA, Saffigna PG (1998) some nutrient dynamics associated with litterfall and litter decomposition in hoop pine plantations of southeast Queensland, Australia. For Ecol Manag 110:343–352
Cadish G, Giller KE (1997) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, p 432
Chadwick DR, Ineson P, Woods C, Piearce TG (1998) Decomposition of Pinus sylvestris litter in litter bags: influence of underlying native litter. Soil Biol Biochem 30:47–55
Chandrasekhara UM (1997) Litter decomposition as an ecosystem service. A Report by KFRI 35–53
Chapman SK, Koch GW (2007) What type of diversity yields synergy during mixed litter decomposition in a natural forest ecosystem? Plant Soil 299:153–162
Coleman DC, Crossley DA (1996) Essentials of soil ecology. Academic Press Inc, Cambridge, p 205
Coleman DC, Blair JM, Eliott ET, Freckman DW (1999) Soil invertebrates. In: Sollins P, Robertson GP, Bledsoe CS, Coleman DC (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 349–377
Cornelissen JHC (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582
Couteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Cox P, Fischer PJ, Anderson JM (1997) Experiments in fungal survival of two common pine litter colonisers. Mycologist 11:55–58
Cox P, Wilkinson SP, Anderson JM (2001) Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinus sylvestris litter. Biol Fert Soils 33:246–251
Crawford RL (1981) Lignin biodegradation and transformation. Wiley, New York, p 137
Crossley DA, Hoglund MP (1962) A litter-bag method for the study of microarthropods inhabiting leaf litter. Ecology 43:571–573. doi:10.2307/1933396
Cuevas E, Medina E (1986) Nutrient dynamics within Amazonian forests: part 1, nutrient flux in fine litter fall and efficiency of nutrient utilization. Oecologia 68:466–472
Cuevas E, Medina E (1988) Nutrient dynamics within Amazonian forests II. Fine rootgrowth, nutrient availability and leaf litter decomposition. Oecologia 76:222–235
D’Souza TM, Merritt CS, Reddy CA (1999) Lignin-modifying enzymes of the white-rot basidiomycete Ganoderma lucidum. Appl Environ Microbiol 65:5307–5313
Das AJ, Battles JJ (2007) The relationship between tree growth patterns and likelihood of mortality: a study of two tree species in the Sierra Nevada. Can J For Res 37:580–597
Daubemire R, Prusso DC (1963) Studies of the decomposition rates of tree litter. Ecology 44:589–592
Dechaine J, Ruan H, Sanchez de Leon Y, Zou X (2005) Correlation between earthworms and plant litter decomposition in a tropical wet forest of Puerto Rico. Pedobiologia 49(6):601–607
Dejonghe W, Boon N, Seghers D, Top EM, Verstraete W (2001) Bio-augmentation of soils by increasing microbial richness: missing links. Environ Microbiol 3:649–657
Devi AS, Yadava PS (2007) Wood and leaf litter decomposition of Dipterocarpus tuberculatus Roxb. In a tropical deciduous forest of Manipur. North East India. Curr Sci 93:243–246
Dilly O, Munch JC (2001) Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biol Biochem 33:921–930
Dilly O, Bloem J, Vos A, Munch JC (2004) Bacterial diversity in agricultural soils during litter decomposition. Appl Environ Microbiol 70:468–474
Dyer ML, Meentemeyer V, Berg B (1990) Apparent controls of mass loss rate of leaf litter on a regional scale. Scand J For Res 5:311–323
Edwards CA, Heath GW (1963) The role of soil animals in breakdown of leafmaterial. In: Doeksen J, Van der Drift J (eds) Soil organisms. North Holland Publishing Co, Amsterdam, pp 76–84
Eriksson KE, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, Berlin, p 407
Esperschutz J, Welzl G, Schreiner K, Buegger F, Munch JC, Schloter M (2011) Incorporation of carbon from decomposing litter of two pioneer plant species into microbial communities of the detritusphere. FEMS Microb 320:48–55
Esperschutz J, Zimmermann C, Dumig A, Welzl G, Buegger F, Elmer M, Munch JC, Schloter M (2013) Dynamics of microbial communities during decomposition of litter from pioneering plants in initial soil ecosystems. Biogeosciences 10:5115–5124. doi:10.5194/bg-10-5115-2013
Fengel D, Wegener G (1983) Wood: chemistry, ultrastructure, reactions. De Gruyter, Berlin, p 613
Finlay BJ, Brown S, Clarika KJ, Esteban GF, Hindle RM, Olmo JL, Rollett A, Vickerman K (2000) Estimating the growth potential of the soil protozoan community. Protist 151:69–80
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fogel R, Cromack K (1977) Effect of habitat and substrate quality on Douglas fir litter decomposition in western Oregon. Can J Bot 55:1632–1640
Frankland JC (1992) Mechanisms in fungal succession. In: Carroll GC, Wicklow DT (eds) The fungal community: its organisation and role in the ecosystem. Marcel Dekker, New York, pp 383–402
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gaudinski JB, Trumbore SE, Davidson EA, Zheng S (2000) Soil carbon cycling in a temperate forest: radio-carbon based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry 51:33–69
Gilbertson RL (1980) Wood-rotting fungi of North America. Mycologia 72:1–49
Giller KE, Gadisch G (1997) Driven by nature: a sense of arrival or departure. CAB International, Wallingford, pp 393–399
Gonzalez G, Seastedt TR (2001) Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82(4):955–964
Gosz JR, Likens GE, Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecol Monogr 43:173–191
Guo EB, Sims REH (1999) Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agr Ecosyst Environ 75:133–140
Gustafson FG (1943) Decomposition of the leaves of some forest trees under field conditions. Plant Phys 18:704–707
Hanson PJ, Swanston CW, Gartnen CT (2005) Reconciling change on Oi- horizon C-14 with mass loss for an oak forest. Soil Sci Soc Am J 69:1492–1502
Harmon ME, Lajtha K (1999) Analysis of detritus and organic horizons for mineral and organic constituents. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 143–165
Harmon ME, Nadelhoffer KJ, Blair JM (1999) Measuring decomposition, nutrient turnover, and stores in plant litter. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 202–240
Hatakka A (2001) Biodegradation of lignin. In: Hofman M, Stein A (eds) Biopolymers vol 1 Lignin, humic substances and coal. Wiley, Weinheim, pp 129–180
Hattenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432
Heath (1966) Biology of plant litter decomposition, vol 1. Academic press, London
Hedin LO (2000) Deposition of nutrients and pollutants to ecosystems. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods of ecosystem science. Springer, New York, pp 265–276
Herrera R, Merida T, Stark N, Jordan CF (1978) Direct phosphorus transfer from dead litter to roots. Naturwissenschaften 65:208–209
Highley TL (1988) Cellulolytic activity of brown-rot and white-rot fungi on solid media. Holzforschung 42:211–216
Higuchi T (1993) Biodegradation mechanism of lignin by white-rot basidiomycetes. J Biotechnol 30:1–8
Hobbie SE (1996) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Horner JD, Gosz JR, Cates RG (1988) The role of carbon-based plant secondary metabolites in decomposition in terrestrial ecosystems. Am Nat 132:869–883
Irmler U (1982) Litter fall and nitrogen turnover in an Amazonian black water inundation forest. Plant Soil 67:355–358
Isaac SR, Nair MA (2005) Biodegradation of leaf litters in the warm humid tropics of Kerala, India. Soil Biol Biochem 37:1656–1664
Jenkinson DS, Bradbury NJ, Coleman K (1994) How the Rothamsted classical experiments have been used to develop and test models for the turnover of carbon and soil nitrogen. Long-term experiments in agricultural and ecological studies. CAB International, Oxon, pp 117–138
Jha DK, Sharma GD, Mishra RR (1992) Soil microbial population numbers and enzyme activities in relation to altitude and forest degradation. Soil Biol Biochem 24:761–767
Jin L, Schultz TP, Nicholas DD (1990) Structural characterization of brown-rotted lignin. Holzforschung 44:133–138
Johansson MB (1994a) Decomposition rates of Scots pine needle litter related to site properties litter quality and climate. Can J For Res 24:1771–1781
Johansson MB (1994b) Decomposition rates of Scots pine needle litter related to site properties, litter quality and climate. Can J For Res 24:1771–1781
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta analysis. For Ecol Manag 140:227–238
Johnson EA, Catley KM (2002) Life in the leaf litter. Centre for Biodiversity and Conservation, New York, p 25
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Karberg NJ, Scott NA, Giardina CP (2008) Methods for estimating litter decomposition. Springer, Berlin, pp 103–111
Kelly JM, Beauchamp JJ (1987) Mass loss and nutrient changes in decomposing upland oak and mesic-hardwood leaf litter. Soil Sci Soc Am J 51:1616–1622
Kirschbaum MUF (1995) The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic carbon storage. Soil Biol Biochem 27:753–760
Kirschbaum MUF (2000) Will changes in soil organic carbon act as a negative or positive feedback on global warming? Biogeochemistry 48(1):21–51
Kjoller A, Struwe S (1992) Functional groups of microfungi in decomposition. In: The fungal community: its organization and role in the ecosystem, vol 2. Marcel Dekker, pp 619–630
Klein C, Dutrow B (2000) Manual of mineral science. John Wiley & Sons Inc, New York
Knapp EB, Elliott LF, Campbell GS (1983) Microbial respiration and growth during the decay of wheat straw. Soil Biol Biochem 15:319–323
Krivtsov V, Liddell K, Bezginova T, Salmond R, Staines HJ, Watling R, Garside A, Thompson JA, Griffiths BS, Brendler A (2005) Forest litter bacteria: relationships with fungi, Microfauna, and litter composition over a winter-spring period. Pol J Ecol 53(3):383–394
Kucera CL (1959) Weathering characteristics of deciduous leaf litter. Ecol 40:485–487
Kumar R, Tapwal A, Baruah DM (2012) Leaf litter decomposition pattern in Dipterocarpus tuberculatus and Dipterocarpus retusus forests of North East India. RJF 6:24–31
Kurihara Y, Kikkawa J (1986) Trophic relations of decomposers. Blackwell Scientific Publications, Melbourne, pp 126–160
Liu Y, Wang S, Wang Q, Zhang J (2010) Effects of mixed-species leaf litter on litter decomposition and soil microbial communities in experimental subtropical plantation forest. J Food Agric Environ 8(3&4):1102–1107
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8(3):315–323
Loreau M (2001) Micobial diversity, producer decomposer interactions and ecosystem processes: a theoretical model. Proc R Soc Lond Ser B 268:303–309
Lueken H, Hutcheon WL, Paul EA (1962) The influence of nitrogen on the decomposition of crop residues in the soil. Can J Soil Sci 42:276–288
Luizao RCC, Bonde TA, Rosswall T (1992) Seasonal variation of soil microbial biomass- the effects of clear felling a tropical rain forest and establishment of pasture in the central Amazon. Soil Biol Biochem 24:805–813
Madge DS (1965) Leaf fall and litter disappearance in a tropical forest. Pedobiol 5:273–288
Mahasneh AM (2001) Bacterial decomposition of Avicennia marina leaf litter from Al-Khor. J Biol Sci 1:717–719
Martin A, Marinissen JCY (1993) Biological and physico-chemical processes in excrements of soil animals. Geoderma 56:331–347
Mattingly GEG, Williams RJB (1962) A note on the chemical analysis of a soil buried since roman times. Eur J Soil, Sci
McKee KL, Faulkner PL (2000) Restoration of biogeochemical function in mangrove forests. Restor Ecol 8(3):247–259
McTiernan KB, Ineson P, Coward PM (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78:527–538
Medina E, Cuevas E (1989) Patterns of nutrient accumulation and release in Amazonian forests of upper Rio Negro basin. In: Mineral nutrients in tropical forest and savanna ecosystems. Blackwell Scientific, Oxford, pp 217–240
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecol 59:465–472
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Mikola P (1960) Comparative experiment on decomposition rates of forest litter in southern and northern Finland. Oikos 11:161–166
Mikola P (1973) Application of mycorrhizal symbiosis in forestry practice: their ecology and physiology. Ectomycorrhizae. Academic Press, New York, pp 383–411
Millar HC, Smith FB, Brown PE (1936) The rate of decomposition of various plant materials in soils. J Am Soc Agron 28:914–923
Minderman G (1968) Addition, decomposition and accumulation of organic matter in forests. J Ecol 56:355–362
Nicolardot B, Recous S, Mary B (2001) Simulation of carbon and nitrogen mineralisation during crop residue decomposition: a simple dynamic model based on the C:N ratio of the residues. Plant Soil 228:83–103
Nilsson T, Daniel G, Kirk TK, Obst JR (1989) Chemistry and microscopy of wood decay by some higher ascomycetes. Holzforschung 43:11–18
Nye PH (1961) Organic matter and nutrient cycles under moist tropical forest. Plant Soil 13:333–346
Olsen JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecol 44:322–331
Ovington (1954) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin
Ovington JD, Madgwick HAI (1957) Afforestation and soil reaction. J Soil Sci 8:141–149
Pascoal C, Cassio F (2004) Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Appl Environ Microbiol 70:5266–5273
Perez-Harguindeguy N, Diaz S, Cornelissen JHC, VenraminiF Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
Persson H (1980) spatial distribution of fine-root growth, mortality and decomposition in a young Scots pine stand in Central Sweden. Oikos 34:77–87
Peterjohn WT, Melillo JM, Steudler PA, Newhrk KM, Bowles FP, Aber JD (1994) Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecol Appl 4:617–625
Prescott CE (1996) Influence of forest floor type on rates of litter decomposition in microcosms. Soil Biol Biochem 28:1319–1325
Prescott CE, Zabek LM, Staley CL, Kabzems R (2000) Decomposition of broad leaf and needle litter in forests of British Columbia: influences of litter type, forest type and litter mixtures. Can J For Res 30:1742–1750
Prosser JI (2002) Molecular and functional diversity in soil micro-organisms. Plant Soil 244:9–17
Rigobelo EC, Nahas E (2004) Seasonal fluctuations of bacterial population and microbial activity in soils cultivated with Eucalyptus and Pinus. Sci Agric 61:88–93
Robertson GP, Paul EA (1999) Decomposition and soil organic matter dynamics. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods of ecosystem science. Springer, New York, pp 104–116
Rochow (1974) Climate and the decomposition rate of-tropical forest litter. UNFAO, Rome
Rosenbrock P, Buscot F, Munch JC (1995) Fungal succession and changes in the fungal degradation potential during the early stages of litter decomposition in black alder forest (Alnusglutinosa(Gaertn.) L.). Eur J Soil Biol 31:1–11
Santa Regina I, Tarazona T (2001) Nutrient cycling in a natural beech forest and adjacent planted pine in northern Spain. Forestry 74:11–28
Sarah EH (1996) Temperature and plant species control over litter decomposition in Alaskan Tundra. Ecol Monogr 66:503–522
Schaefer MA, Schauermann J (1990) The soil fauna of beech forests: comparisonbetween a mull and a moder soil. Pedobiologia 34:299–314
Schimel (1995) Terrestrial ecosystems and the carbon cycle. Glob Change Biol 1:77–91
Schlesinger WH (1997) Biogeochemistry, an analysis of global change, 2nd edn. Academic Press, New York
Scholle G, Wolters V, Joergensen RG (1992) Effects of mesofauna exclusion on the microbial biomass in two modern profiles. Biol Fert Soils 12:253–260
Scowcroft PG, Turner DR, Vitousek PM (2000) Decomposition of Metrosideros polymorpha leaf litter along elevational gradients in Hawaii. Glob Change Biol 6:73–85
Simpson AJ, Myrna JS, Smith E, Kelleher P (2007) Microbially derived inputs to soil organic matter: are current estimates too low? Environ Sci Technol 41:8070–8076
Singh KP (1969) Studies in decomposition of leaf litter of important trees of tropical deciduous forests at varanasi. Trop Ecol 10:292–311
Singh JS, Gupta SR (1977) Plant decomposition and soil respirations in terrestrial ecosystems. Bot Rev 43:449–460
Swarnalatha B, Reddy MV (2011) Leaf litter breakdown and nutrient release in three tree plantations compared with a natural degraded forest on the coromandel coast (puducherry, india). Ecotropica 17:39–51
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. In: Anderson DJ, Greig-smith P, Pitelka FA (eds) Studies in ecology, vol 5. University of California Press, Berkeley, pp 167–219
Teuben A (1991) Nutrient availability and interactions between soil arthropods duringdecomposition of coniferous litter: a mesocosm study. Biol Fert Soils 10:256–266
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci 81:2280–2284
Torsvik V, Goksoyr J, Dane FL, Sorheim R, Michaelsen J, Solte K (1994) Use of DNA analysis to determine the diversity of microbial communities. In: Ritz K, Dighton J, Giller KE (eds) Beyond the biomass. Wiley, Newyork, pp 39–48
Tripathi G, Deora R, Singh J (2010) Biological, chemical and biochemical dynamics during litter decomposition at different depths in arable soil. J Ecol Nat Environ 2(3):038–051
Verhoef HA, Brussaard L (1990) Decomposition and nitrogen mineralization in natural and agro-ecosystems: the contribution of soil animals. Biogeochemistry 11:175–211
Vesterdal L (1999) Influence of soil type on mass loss and nutrient release from decomposing foliage litter of beech and Norway spruce. Can J For Res 29:95–105
Viljoen JA, Fred ED, Peterson WH (1926) The fermentation of cellulose by thermophilic bacteria. J Agric Sci 16:1–17
Vitousek PM, Turner DR, Parton WJ, Stanford RL (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawaii: patterns, mechanisms, and models. Ecology 75(2):418–429
Wachendorf C, Irmler U, Blume HP (1997) Relationship between litter fauna and chemical changes during litter decomposition under different moisture conditions. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, pp 135–144
Wang Y, Zhang ZS, Ruan JS, Wang YM, Ali SM (1999) Investigation of actinomycete diversity in the tropical rainforests of Singapore. J Ind Microbiol Biotechnol 23:178–187
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which doesnot support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Waring RH, Schleslnger WH (1985) Forest ecosystems: concepts and management. Academic Press, Orlando
Wedderburn ME, Carter J (1999) Litter decomposition by four functional tree types for use in silvopastoral systems. Soil Biol Biochem 31:455–461
Wiegel J, Dykstra M (1984) Clostridium thermocellum: adhesion and sporulation while adhered to cellulose and hemicellulose. Appl Microbiol Biotechnol 20:59–65
Willams ST, Gray TRG (1974) Decomposition of litter on the soil surface. In: Dickinson CH, Pugh GJF (eds) Biology of plant litter decomposition, vol 2. GJF, pp 611–632
Wolter KE, Highley TL, Evans FJ (1980) A unique polysaccharide- and glycoside-degrading enzyme complex from the wood decay fungus Poria placenta. Biochem Biophys Res Commun 97:1499–1504
Wood M (1995) The role of bacteria and actinomycetes in litter decomposition in the tropics. Soil Organ Litter Decompos Trop 13–37
Xu XN, Hirata E (2002) Forest floor mass and litterfall in Pinus luchuensis plantations with and without broad-leaved trees. For Ecol Manage 157:165–173
Acknowledgements
The authors are grateful to the anonymous reviewers for their valuable suggestions and guidance in shaping this article. The first author also acknowledges the Junior Research Fellowship from the Department of Environment and Climate Change, Government of Kerala.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna, M.P., Mohan, M. Litter decomposition in forest ecosystems: a review. Energ. Ecol. Environ. 2, 236–249 (2017). https://doi.org/10.1007/s40974-017-0064-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-017-0064-9